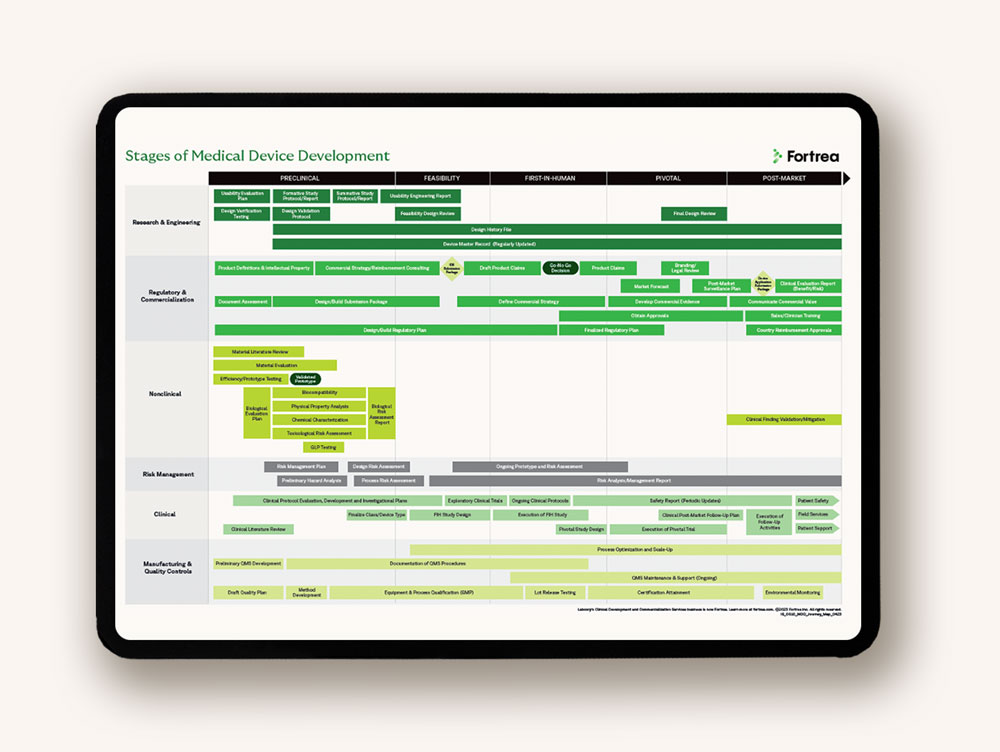
Developing medical devices demands a particular set of skills. Global regulatory requirements are medical device specific, as are the specifics of protocol design and study conduct, quality systems demands and commercialization considerations. That's why Fortrea established a dedicated medical device division as part of our global CRO to consult and support you with the insights and experience needed to maximize the potential of your device.
Our partnership enables the scale and reach of our established global organization, giving you the strength and flexibility you need to proceed with confidence.