Zelltherapien
Pioneering precision medicine development
We deliver the expertise and strategic guidance you need to successfully navigate the complexities of cell therapy development. From initial patient screening to long-term follow-up, we proactively manage risks to accelerate your path to market.
Deep expertise in cell therapy trials
Depend on our industry-leading experience to navigate regulatory hurdles, optimize trial design and ensure patient safety across all trial phases.
Global reach with local support
Benefit from our worldwide network of specialized sites and local experts, ensuring seamless trial execution and patient recruitment across key regions.
Comprehensive risk management
Our proactive approach identifies potential challenges early, minimizing delays and ensuring your cell therapy reaches patients faster.
From your “Eureka!” moment to market access
We’re at the forefront of cell therapy research, with fine-tuned expertise to enable breakthrough programs—including all six FDA-approved CAR T-cell therapies.
We know that successfully completing a cell therapy program-whether CAR T, TCR, TIL or other cell constructs- requires managing inherent risks or unexpected delays. From patient screening, through cell product infusion and long-term follow-up, we take preemptive steps to minimize risks and accelerate your development journey.
Our experience spans multiple modalities, including immune cell/stem cell, autologous/allogeneic, modified/non-modified, CAR T and adeno-associated viruses. Let's turn your paradigm-shifting ideas into treatments.

Cell therapy experience that matters
In the last 5 years, we have supported:
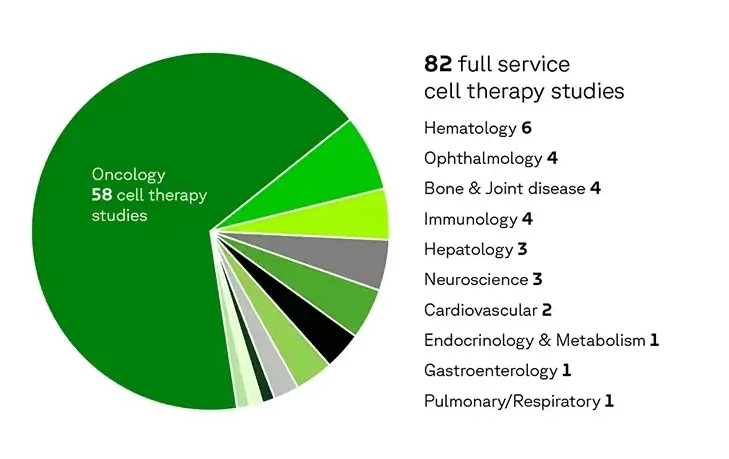
Our cell therapy experience
Our experience spans a range of therapeutic areas, with 71 % in oncology indications.
Seamless delivery for your next trial
Working as an extension of your team, our multidisciplinary approach ensures comprehensive support to navigate the complexities of your gene therapy program with precision and expertise.
-
Scientific and medical expertise
Put decades of expertise in development behind your program. With deep knowledge of how these complex therapies impact the conduct of a clinical trial, we support:
- Protokollentwicklung
- Apheresis
- Complex cell therapy product logistics
- Long-term follow-up (LTFU)
- Companion diagnostics development
- GMO and ATMP global regulatory compliance
- Vendor qualification
- Medical monitoring
-
Global operational solutions
Tailored solutions and cutting-edge technologies to optimize your clinical trial through:
- Logistics support for autologous CAR T-cell therapies
- Streamlined site selection, activation and study setup
- Precise regulatory guidance, including GMO pathways and early engagement strategies (INTERACT, pre-IND)
- Reduced patient burden in LTFU studies
- Smooth regulatory journey, especially in rare diseases and pediatrics
- Data-driven site and patient engagement strategy
-
Customized training
Comprehensive training programs designed for complex and rapidly evolving technologies:
- Targeted, study-specific training
- Formal cell and gene therapy training to equip your team for trial demands
Related indications
The clock is ticking. Tap into our multidisciplinary experts to get the most from your cell therapy study. Together, we'll improve your odds of success and address urgent, unmet medical needs.
Explore our related areas of expertise.

